Synthesis of Novel Nano-Strawberry TiO2 Structures with the Aid of Microwave Inverter System: Growth Time Effect on Optical Absorption Intensity
DOI:
https://doi.org/10.18502/keg.v1i1.507Abstract
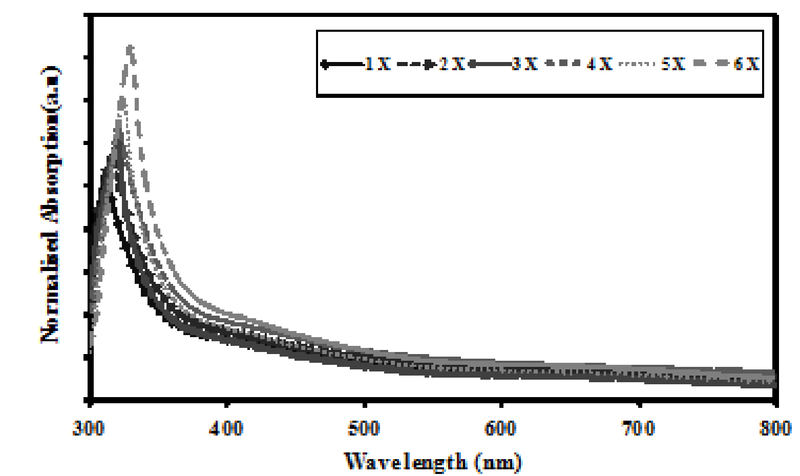
A novel anatase TiO2 with nanostrawberry-like structure with high porosity has been synthesised on ITO, with the aid of microwave power in a very short duration of 6 minutes. The growth of these novel TiO2nanostructures on ITO is attained stoichiometrically by using ammonium hexafluoro titanate, Hexamethylenetetramine, and Boric acid as precursor, capping agent, and reducing agent, respectively. Optical absorption intensity and thickness of these nanostructure layers can be varied by the growth time. A highly porous, 2.25 µm thickest layer has been successfully synthesised on ITO, and the average diameter of these nanostructures was found approximately 70±2.5nm. These highly porous nanostructures are expected to be good candidate for photocatlysis applications and efficient photovoltaic performances of dye sensitised solar cells.
References
S. K. M. Saad, et al., Porous (001)-faceted Zn-doped anatase TiO2 nanowalls and their heterogeneous photocatalytic characterization, RSC Advances, 4, 57054–57063, (2014).
J. Nisar, Z. Topalian, A. De Sarkar, L. Österlund, and R. Ahuja, TiO2-based gas sensor: a possible application to SO2, ACS Appl Mater Interfaces, 5, 8516–8522, (2013).
A. F. Khan, M. Mehmood, T. Ali, and H. Fayaz, Structural and optical studies of nanostructured TiO2–Ge multi-layer thin films, Thin Solid Films, 536, 220–228, (2013).
J. Tang, J. R. Durrant, and D. R. Klug, Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photo holes, importance of charge carrier dynamics, and evidence for four-hole chemistry, J Am Chem Soc, 130, 13885–13891, (2008).
A. A. Umar, S. Nafisah, S. KM. Saad, S. T. Tan, A. Balouch, M. M. Salleh, and M. Oyama, Poriferous microtablet of anatase TiO2 growth on an ITO surface for high-efficiency dye-sensitized solar cells, Sol Energy Mater Sol Cells, 122, 174–182, (2014).
M. Lazzeri, A. Vittadini, and A. Selloni, Structure and energetics of stoichiometric TiO2 anatase surfaces., Physical Review B, 36, no. 15, (2001).
M. M. Byranvand, et al., A Review On Synthesis Of Nano-TiO2 Via Different Methods, Journal of Nanostructures, 1–9, (2013).
A. A. Umar, M. Y. A. Rahman, S. K. M. Saad, M. M. Salleh, and M. Oyama, Preparation of grass-like TiO2 nanostructure thin films: effect of growth temperature, Appl Surf Sci, 270, 109–114, (2013).
Y. U. Bykov, K. I. Rybakov, and V. E. Semenov, High-temperature microwave processing of materials, Journal of Physics D: Applied Physics, 34, no. 13, p. R55, (2001).
E. T. Thostenson and T.-W. Chou, Microwave processing: fundamentals and applications, Compos, Part A Appl Sci Manuf, 30, 1055–1071, (1999).
A. A. Salema, Y. K. Yeow, K. Ishaque, F. N. Ani, M. T. Afzal, and A. Hassan, Dielectric properties and microwave heating of oil palm biomass and biochar, Ind Crops Prod, 50, 366–374, (2013).
Y. Tao, C.-Y. Wu, and D. W. Mazyck, Microwave-assisted preparation of TiO2/activated carbon composite photocatalyst for removal of methanol in humid air streams, Ind Eng Chem Res, 45, 5110–5116, (2006).
B. S. Shirke, P. V. Korake, P. P. Hankare, S. R. Bamane, and K. M. Garadkar, Synthesis and characterization of pure anatase TiO2 nanoparticles, J Mater Sci Mater Electron, 22, 821–824, (2011).