Submission Guidelines
All manuscripts must be submitted online through this website.
This copyright form must also be submitted.
First time users will have to register at this site. Registration is free but mandatory. Registered authors can keep track of their articles after logging into the site using their username and password. Authors do not have to pay for the submission, processing or publication of articles. If you experience any problems, please contact the editorial office by e-mail at labbafi@gmail.com.
The submitted manuscripts that are not as per the Submission Guidelines would be returned to the authors for technical correction, before they undergo editorial/ peer-review. Generally, the manuscript should be submitted in the form of separate files
File 1: Title Page
This file should provide
The type of manuscript (Original Article, Review Article, Case Report, Letter to Editor, Surgical Technique, etc.), the title of the manuscript, running title, names of all authors/ contributors (with their highest academic degrees, designation and affiliations, ORCID) and name(s) of the department(s) and/ or institution(s) to which the work should be credited. All information which can reveal your identity should be here. Use text/rtf/doc files. Do not zip the files.
The total number of pages, the total number of figures and word counts separately for the abstract and for the text (excluding the references, tables and abstract).
Source(s) of support in the form of grants, equipment, drugs, or all of these;
Acknowledgement, if any. One or more statements should specify
- Contributions that need acknowledging but do not justify authorship, such as general support by a departmental chair;
- Acknowledgements of technical help; and
- Acknowledgements of financial and material support, which should specify the nature of the support. This should be included in the title page of the manuscript and not in the main article file.
If the manuscript was presented as part of a meeting, the organization, place, and exact date on which it was read. A full statement to the editor about all submissions and previous reports that might be regarded as redundant publications of the same or very similar work. Any such work should be referred to specifically, and referenced in the new paper. Copies of such material should be included with the submitted paper, to help the editor decide how to handle the matter.
Registration number in case of a clinical trial and where it is registered (name of the registry and its URL)
Conflicts of Interest of each author/ contributor. A statement of financial or other relationships that might lead to a conflict of interest, if that information is not included in the manuscript itself or in an author's form
Criteria for inclusion in the authors’/ contributors’ list
A statement that the manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work if that information is not provided in another form (see below); and
The name, address, postal code, e-mail, and telephone number of the corresponding author, who is responsible for communicating with the other authors about revisions and final approval of the proofs, if that information is not included in the manuscript itself.
If the manuscript has been submitted to the preprint platform, the following information should be clearly disclosed:
- The name of the platform, DOI, and link should be clearly indicated.
- Confirm that the preprint version has not been formally peer-reviewed.
- Confirm that the preprint server that the preprint was published on will also link to the definitive version of the article to ensure that all readers can quickly and easily find the most recent, peer-reviewed version of the work.
File 2: Main Text
The main text of the article, beginning from the abstract to references, should be in this file. The file must not contain any mention of the authors' names or initials or the institution at which the study was done, or acknowledgements. Page headers/running titles can include the title but not the authors' names.
Manuscripts not in compliance with the journal's blinding policy will be returned to the corresponding author. Use rtf/doc files. Do not zip the files. Limit the file size to 1 MB. Please do not incorporate images in the file. If the file size is large, graphs can be submitted as images separately without incorporating them in the article file to reduce the size of the file. The pages should be numbered consecutively, beginning with the first page of the blinded article file. Tables should be provided at the end of this file.
File 3: Figures Legends
Maximum 600 characters for each figure.
File 4: Figures
Submit good-quality colour figures. Each figure should be less than 2 MB in size. The size of the figure can be reduced by decreasing the actual height and width of the figures (keep up to 1600 x 1200 pixels or 5-6 inches). Figures can be submitted as jpeg files. Please do not zip the files. Legends for the figures should be included at the end of the article file.
Preparation of All Types of Manuscripts
Manuscripts must be prepared in accordance with "Uniform requirements for Manuscripts submitted to Biomedical Journals" developed by the International Committee of Medical Journal Editors (October 2008). The uniform requirements and specific requirements of the Journal of Ophthalmic and Vision Research are summarized below. Before submitting a manuscript, contributors are requested to check for the latest instructions available.
Journal of Ophthalmic and Vision Research accepts manuscripts written in American English.
Copies of Any Permission(s)
It is the responsibility of authors/ contributors to obtain permission to reproduce any copyrighted material. A copy of the permission obtained must accompany the manuscript. Copies of any and all published articles or other manuscripts in preparation or submitted elsewhere that are related to the manuscript must also accompany the manuscript.
Statistical Analysis
Whenever possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Authors should report losses to observation (such as dropouts from a clinical trial). When data are summarized in the Results section, specify the statistical methods used to analyze them. Avoid non-technical uses of technical terms in statistics, such as 'random' (which implies a randomizing device), 'normal', 'significant', 'correlations', and 'sample'. Define statistical terms, abbreviations, and most symbols. Specify the computer software used. Use upper italics (P 0.048). For all P values, include the exact value and not less than 0.05 or 0.001. Mean differences in continuous variables, proportions in categorical variables and relative risks, including odds ratios and hazard ratios, should be accompanied by their confidence intervals.
Results
Present your results in a logical sequence in the text, tables, and illustrations, giving the main or most important findings first. Do not repeat in the text all the data in the tables or illustrations; emphasize or summarize only important observations. Extra- or supplementary materials and technical detail can be placed in an appendix where it will be accessible but will not interrupt the flow of the text; alternatively, it can be published only in the electronic version of the journal.
When data are summarized in the Results section, give numeric results not only as derivatives (for example, percentages) but also as the absolute numbers from which the derivatives were calculated, and specify the statistical methods used to analyze them. Restrict tables and figures to those needed to explain the argument of the paper and assess its support. Use graphs as an alternative to tables with many entries; do not duplicate data in graphs and tables. Where scientifically appropriate, analyses of the data by variables such as age and sex should be included.
Discussion
Include a summary of key findings (primary outcome measures, secondary outcome measures, and results as they relate to a prior hypothesis). Strengths and limitations of the study (study question, study design, data collection, analysis and interpretation). Interpretation and implications in the context of the totality of evidence (is there a systematic review to refer to, if not, could one be reasonably done here and now? what this study adds to the available evidence, effects on patient care and health policy, and possible mechanisms). Controversies raised by this study. Future research directions (for this particular research collaboration, underlying mechanisms, clinical research).
Do not repeat in detail data or other material given in the Introduction or the Results section. In particular, contributors should avoid making statements on economic benefits and costs unless their manuscript includes economic data and analyses. Avoid claiming priority and alluding to work that has not been completed. New hypotheses may be stated if needed. However, they should be clearly labelled as such.
Reference
All manuscripts should be accompanied by relevant references. The referencing style must be Vancouver.
Tables
- Tables should be self-explanatory and should not duplicate textual material.
- Tables with more than 10 columns and 25 rows are not acceptable.
- Number tables, in Arabic numerals, consecutively in the order of their first citation in the text and supply a brief title for each.
- Place explanatory matter in the footnotes, not in the heading.
- Spell out all abbreviations that are used in each table in footnotes (for instance: IOP, intraocular pressure).
- Obtain permission for all fully borrowed, adapted, and modified tables and provide a credit line in the footnote.
- For footnotes, use the following symbols in this sequence: *, †, ‡, §, ||,¶, **, ††, ‡‡
- Tables with their legends should be provided at the end of the text after the references. The tables, along with their number, should be cited at the relevant place in the text.
- Do not include the tables in the First page or Article files; please upload each table as a multimedia file.
Figures
- Upload the figures in JPEG format. The file size should be within 10 MB in size while uploading.
- Figures should be numbered consecutively according to the order in which they have been first cited in the text.
- Labels, numbers, and symbols should be clear and of uniform size. The lettering for figures should be large enough to be legible after reduction to fit the width of a printed column.
- Symbols, arrows, or letters used in photomicrographs should contrast with the background and should be marked neatly with transfer type or by tissue overlay and not by pen.
- Titles and detailed explanations belong in the legends for illustrations, not on the illustrations themselves.
- When graphs, scatter grams or histograms are submitted, the numerical data on which they are based should also be supplied.
- The photographs and figures should be trimmed to remove all the unwanted areas.
- If photographs of individuals are used, their pictures must be accompanied by written permission to use the photograph.
- If a figure has been published elsewhere, acknowledge the original source and submit written permission from the copyright holder to reproduce the material. A credit line should appear in the legend for such figures.
- Figure legends: Provide figure legends in a maximum of 600 characters. When symbols, arrows, numbers, or letters are used to identify parts of the illustrations, identify and explain each one in the legend. Explain the internal scale (magnification) and identify the method of staining in photomicrographs.
Final figures for print production: Send sharp, glossy, un-mounted, colour photographic prints, with a height of 4 inches and width of 6 inches at the time of submitting the revised manuscript. Printouts of digital photographs are not acceptable. If digital images are the only source of images, ensure that the image has a minimum resolution of 300 dpi or 1800 x 1600 pixels in TIFF format. The Journal reserves the right to crop, rotate, reduce, or enlarge the photographs to an acceptable size.
Original Articles
These include randomized controlled trials, intervention studies, studies of screening and diagnostic tests, outcome studies, cost-effectiveness analyses, case-control series, and surveys with high response rates. The text of original articles amounting to up to 3500 words (excluding abstract, references and tables) should be divided into sections with the headings Abstract, Keywords, Introduction, Methods, Results, Discussion, References, Tables and Figure Legends.
Abstract
It should be divided into sections with the headings “Purpose, Methods, Results, Conclusion,” and it should be provided in a maximum of 250 words.
Keywords
Three to six keywords should be included according to the MeSH terms.
Introduction
State the purpose and summarize the rationale for the study or observation.
Methods
It should include and describe the following aspect
- Ethics
When reporting studies on human beings, indicate whether the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2000 (available at http://www.wma.net/e/policy/17-c_e.html). For prospective studies involving human participants, authors are expected to mention the approval of (the regional/ national/ institutional or independent Ethics Committee or Review Board, obtaining informed consent from adult research participants and obtaining assent for children aged over 7 years participating in the trial. The age beyond which an asset would be required could vary as per regional and/ or national guidelines. Ensure the confidentiality of subjects by desisting from mentioning participants’ names, initials or hospital numbers, especially in illustrative material. When reporting experiments on animals, indicate whether the institutions or a national research council’s guide for, or any national law on the care and use of laboratory animals was followed.
Evidence for approval by a local Ethics Committee (for both human as well as animal studies) must be supplied by the authors on demand. Animal experimental procedures should be as humane as possible, and the details of anaesthetics and analgesics used should be clearly stated. The ethical standards of experiments must be in accordance with the guidelines provided by the CPCSEA and World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans for Studies involving experimental animals and human beings, respectively). The journal will not consider any paper which is ethically unacceptable. A statement on ethics committee permission and ethical practices must be included in all research articles under the ‘Methods’ section.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations'. If any of these sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
- Funding
- Conflicts of interest/Competing interests
- Availability of data and material
- Ethics approval
- Consent to participate
- Consent for publication (According to ICMJE Recommendations for protection of research participants)
- Protection of Research Participants (https://www.care-statement.org/)
Study Design
Selection and Description of Participants: Describe your selection of the observational or experimental participants (patients or laboratory animals, including controls) clearly, including eligibility and exclusion criteria and a description of the source population. Technical information: Identify the methods, apparatus (give the manufacturer's name and address in parentheses), and procedures in sufficient detail to allow other workers to reproduce the results. Give references to established methods, including statistical methods (see below); provide references and brief descriptions for methods that have been published but are not well known; describe new or substantially modified methods, give reasons for using them, and evaluate their limitations. Identify precisely all drugs and chemicals used, including generic name(s), dose(s), and route(s) of administration.
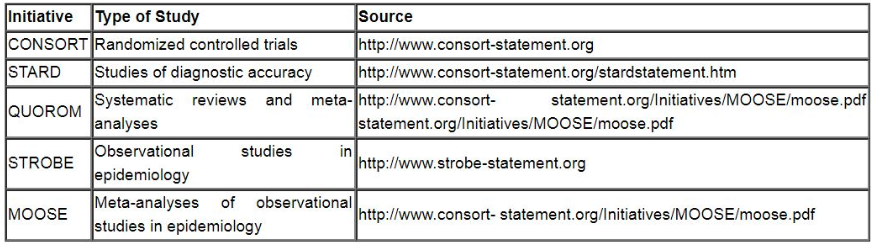
Reports of randomized clinical trials should present information on all major study elements, including the protocol, assignment of interventions (methods of randomization, concealment of allocation to treatment groups), and the method of masking (blinding), based on the CONSORT Statement (http://www.consort-statement.org).
Reporting Guidelines for Specific Study Designs:

Review Articles
It is expected that these articles would be written by individuals who have done substantial work on the subject or are considered experts in the field. A short summary of the work done by the contributor(s) in the field of review should accompany the manuscript.
The word count is variable, depending on the content and type of submission. The manuscript should have an unstructured abstract (maximum 250 words) representing an accurate summary of the article. The section titles would depend upon the topic reviewed. Authors submitting review articles should include a section describing the methods used for locating, selecting, extracting, and synthesizing data. These methods should also be summarized in the abstract.
The journal expects the contributors to give post-publication updates on the subject of review. The update should be brief, covering the advances in the field after the publication of the article and should be sent as a letter to the editor, as and when major development occurs in the field.
Perspective
Perspectives follow the same formatting guidelines as Review Articles but are more prospective and speculative, and may take a narrower field of view. This type of manuscript should contain novel information of significant interest to ophthalmologists and is intended to stimulate discussion and new experimental approaches.
Case Reports
New, interesting and rare cases can be reported. They should be unique, describing a great diagnostic or therapeutic challenge and providing a learning point for the readers. Cases with clinical significance or implications will be given priority. These communications could be of up to 1200 words (excluding abstract and references) and should have the following headings: Abstract, Keywords, Introduction, Case Report, Discussion, References (up to 15 references), Tables and Legends in that order.
The abstract should be divided into sections with the headings “Purpose, Case Report, and Conclusion,” and it should be provided in a maximum of 150 words.
Surgical Technique
New surgical techniques can be arranged to be presented, accompanied by a complete description of the Introduction, Surgical Method, Results, and Discussion.
These manuscripts should be provided in a maximum of 1500 words, excluding the abstract and 15 references.
Unstructured Abstracts should be a maximum of 200 words.
Photo Essays
Images and photographs of interesting or educational content can be presented as photo essays. The manuscript should not exceed 500 words and a maximum of 5 references.
Letter to Editor
These should be short and decisive observations. They should preferably be related to articles previously published in the Journal or views expressed in the journal. They should not be preliminary observations that need a later paper for validation. The letter could have up to 500 words and 5 references. It could be generally authored by not more than four authors.
Other
Clinical Practice Guidelines, Challenging Cases and News.
Protection of Patients' Rights to Privacy
Identifying information should not be published in written descriptions, photographs, sonograms, CT scans, etc., and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian, wherever applicable) gives informed consent for publication. Authors should remove patients' names from figures unless they have obtained informed consent from the patients. The journal abides by ICMJE guidelines:
- Authors, not the journals nor the publisher, need to obtain the patient consent form before the publication and have the form properly archived. The consent forms are not to be uploaded with the cover letter or sent through email to editorial or publisher offices.
- If the manuscript contains patient images that preclude anonymity, or a description that has an obvious indication of the identity of the patient, a statement about obtaining informed patient consent should be indicated in the manuscript.
Sending a Revised Manuscript
The revised version of the manuscript should be submitted online in a manner similar to that used for the submission of the manuscript for the first time. However, there is no need to submit the “First Page” or “Cover Letter” file while submitting a revised version. When submitting a revised manuscript, contributors are requested to include, the ‘referees’ remarks along with point-to-point clarification at the beginning of the revised file itself. In addition, they are expected to mark the changes as underlined or coloured text in the article.
Reprints and Proofs
Journal provides no free printed reprints. Authors can purchase reprints, payment for which should be done at the time of submitting the proofs.
Checklist
Cover letter
- Signed by all contributors
- Previous publications or presentations mentioned
- Source of funding mentioned
- Conflicts of interest disclosed
Authors
- Last name and given name provided along with middle name initials (where applicable)
- Author for correspondence, with the e-mail address provided
- The number of contributors is restricted as per the instructions
- Identity not revealed in the paper except on the title page (e.g. name of the institute in Methods, citing previous study as 'our study', names on figure labels, name of the institute in photographs, etc.)
Presentation and Format
- Arial font (Title page and Main text)
- Double spacing
- Margins 2.5 cm from all four sides
- Page numbers included at the bottom
- The title page contains authors’ first and family names, affiliations, ORCID, correspondence information
- Running title provided (not more than 50 characters)
- Abstract provided according to the journal's instructions
- Keywords provided according to the MeSH terms
- The references cited in the text should be after punctuation marks, in superscript with square bracket
- References according to the journal's instructions, punctuation marks checked
- Send the article file without ‘Track Changes'
Language and Grammar
- Uniformly American English
- Write the full term for each abbreviation at its first use in the title, abstract, keywords and text separately unless it is a standard unit of measure. Numerals from 1 to 10 are spelt out
- Numerals at the beginning of the sentence are spelt out
- Check the manuscript for spelling, grammar and punctuation errors
- If a product is cited, supply complete manufacturer's information (product name and model, manufacturer, city and state/country)
- Measurements’ units should be in accordance with the International System of Units
- Species names should be in italics
Tables and Figures
- No repetition of data in tables and graphs and in text
- Actual numbers from which graphs are drawn provided
- Figures necessary and of good quality (colour)
- Table and figure numbers in Arabic letters (not Roman)
- Labels pasted on the back of the photographs (no names written)
- Figure legends provided (maximum 600 characters)
- Patients' privacy maintained (if not permission taken)
- A credit note for borrowed figures/tables provided
- Spell out each abbreviation used in the table/figure as a footnote or at the end of the figure legends

